This is the Shimadzu GC-2010 Plus, our newest gas chromatograph:
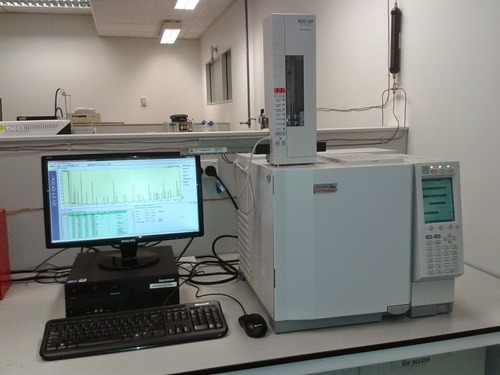
The tower mounted on top is the AOC-20i autoinjector. We also have another two GC-210s with autoinjectors and a pair of venerable GC-17As.

These instruments are used for quantitative analysis of small biomolecules such as fatty acids, amino acids and sugars in organisms, biofluids and food products as well as for profiling the volatile compounds associated with food flavours by Solid Phase Micro Extraction [SPME].
We have a wide range of columns to choose from but two phases that seem to separate most analytes are 5% phenyl, such as Zebron ZB-5, for less polar compounds and wax columns, such as the Zebron DB-WAX, for more polar things. The GC-2010 Plus is fitted with a Restek FAMEWAX column for Fatty Acid Methyl Ester [FAME] analysis and is routinely used to separate and quantify 36 different FAMEs.
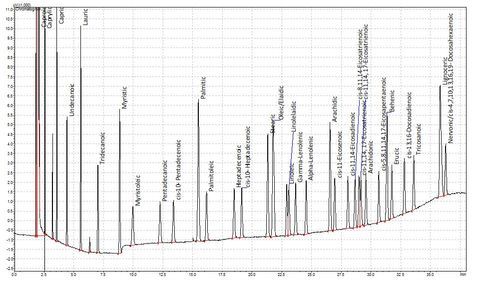
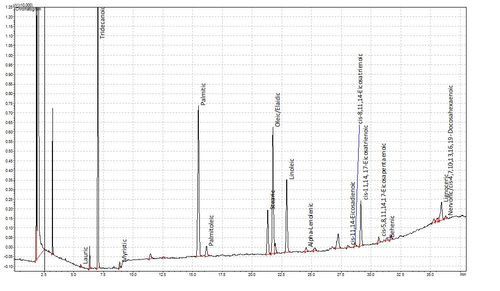
Fatty acids are not volatile in the GC and so cannot be analysed natively. A while ago though, some clever chaps realised that you could esterify fatty acids to make them volatile. This is hugely advantageous as fatty acids are not especially amenable to liquid chromatography either. This method has been refined over the years and we now use a one-tube esterification and extraction method based on de La Cruz Garcia et al (2000). The plots below show a chromatogram of a Supelco FAME standard and below that the FAMEs from a sample of human plasma. Tridecanoic acid is the internal standard.
Another application of our GCs is to analyse sugars. Sugars are even harder to analyse than fatty acids because they are so polar, they do not absorb light strongly and they are not volatile. We use the method of Blakeney et al (1983) to convert sugars from non-volatile compounds to volatile alditol acetates amenable to GC analysis. Here is a chromatogram from our GC-MS showing peaks for ten alditol acetylates in a standard mixture:
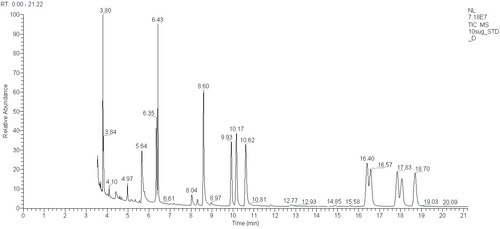
sugar and retention time (mins)
- erythritol 6.43
- rhamnose 9.93
- fucose 10.17
- xylose 10.62
- allose 16.40
- inositol 16.57
- glucose 17.83
- galactose 18.06
- mannose 18.70
References
Blakeney A.B., Harris P.J., Henry R.J., Stone B.A. (1983). A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydrate Research 113:2 p291–299
de La Cruz Garcia, Lopez Hernandez, Simal Lozano (2000). Gas chromatographic determination of the fatty-acid content of heat-treated green beans. Journal of Chromatography A. 891:2 p367–370


[…] as sugars are notoriously hard targets to analyse chromatographically. We currently use a gas chromatographic method which involves a quite lengthy and fiddly acetylation procedure but I’ve found a […]
LikeLike